Pupillary dilation reflex and behavioural pain scale: Study of diagnostic test
Abstract
Background
The objective of this study was to assess the value of the pupillary dilation reflex as an assessment pain tool in critically ill patients. It is important to continue working for the well-being and security of critically ill patients.
Methods
We studied the diagnostic accuracy of the pupillary dilation reflex against the Behavioral Pain Scale. Inclusion criteria were: age greater than 18, receiving mechanical ventilation, with a basal score of the Behavioural Pain Scale of three and a Richmond Agitation and Sedation score between −1 and −4. We studied the responses to a non-painful stimulus, four calibrated stimuli, after a tracheal aspiration and with and without pain. The receiver operating curve was plotted and we calculated the area under the curve. We identified the cut-off points showing the highest sensitivity and specificity and studied diagnostic performance based on negative predictive value, positive predictive value, and accuracy. These were reported with their 95% confidence intervals.
Results
183 measurements were performed. An AUC of 0.88(95% CI 0.83–0.94) was obtained. The pupillary dilation reflex of 11.5% had a sensitivity of 89.8%(95% CI 78.2–95.6) and a specificity of 78.4%(95% CI 70.6–84.5) with an accuracy of 81.4(75.2–86.4). The pupillary dilation reflex detected nociceptive pain response in 15.8% of the measurements that did not show pain according to the Behavioural Pain Scale.
Conclusions
Pupillometry may be a valid alternative for identifying pain in critically ill patients.
Keywords
Critical illness
Intensive care
Pain assessment
Reflex
Pupillary
Implications for clinical practice
Pain assessment in the critically ill patient is a complex area of care
Pupillometry could be a tool to detect the presence autonomic responses to painful procedures in patients with limited or abolished behavioural responses.
Pupillometry could be a useful tool for the assessment of pain and the need for analgesia in critically ill patients.
The use of pupillometry in clinical practice could be helpful in decision making by improving the standardisation of pain protocols and drug management
Introduction
Pain is a significant problem in critically ill patients and is strongly related to the clinical context (Puntillo et al., 2014). The incidence of pain in intensive care units (ICUs) can reach 70 % (Robleda et al., 2016). It is among the most unpleasant lasting memories of the time in the ICU (Rotondi et al., 2002). Inadequate pain management triggers anxiety (Puntillo et al., 2010), hyperadrenergic symptoms, posttraumatic stress syndrome (Rawal et al., 2017), and chronic pain (Puntillo and Naidu, 2016). Conversely, over-sensitization to pain can lead to excessive use of sedatives and analgesics, developing ileus and hypoactive delirium and prolonged mechanical ventilation times, and ICU stays (Pavone et al., 2021). It must be avoided risk factors that trigger side effects in patients by ensuring the well-being of all patients (Schittek et al., 2021). Pain assessment and management should be an ethical obligation in the daily clinical practice of healthcare workers.
Many critically ill patients cannot express their pain. Clinical practice guidelines for managing pain, agitation, and delirium in adult patients in ICU emphasize the importance of implementing strategies that optimize critically ill patient pain management (Barr et al., 2013, Devlin et al., 2018). Behavioural scales have demonstrated optimal psychometric properties. However, their validity decreases in specific patient groups (Gélinas and Johnston, 2007, Payen et al., 2001). Pain assessment is a complex clinical situation, usually approached through critical thinking. Critical care nurses’ decision-making about pain is insufficient, sometimes ineffective and can be a determining factor in the way the patient’s pain is dealt with (Rababa and Al-Rawashdeh, 2021).
Currently, the performance of tools that measure the responses of the autonomic nervous system to nociceptive stimulation as an indicator of pain in surgical patients is being studied (Chanques et al., 2017, Martini et al., 2015). Non-invasive devices allow continuous monitoring of cardiac and cutaneous sympathetic responses. However, this parameter may be distorted by the patient’s hemodynamic situation, the effect of inotropic drugs, or the appearance of arrhythmias. Therefore, the results may not apply to patients admitted to the ICU.
The pupillary dilation reflex (PDR), an autonomous physiological response to nociception, could be an objective indicator of the presence of pain in sedated patients. The size of a pupil has a maximum contraction size of 1.5 mm and can increase in diameter up to 9 mm in darkness. Electrical or mechanical stimulation of the Aσ and C fibres increases pupil size almost three times the basal pupil size. However, these changes are difficult to appreciate by visual inspection (Larson and Behrends, 2015). The video pupillometer is a non-invasive technique, a device with infrared light that allows exploration of these changes in a dark environment. It measures the pupillary reflex after nociceptive stimulation (Constant et al., 2006, Guglielminotti et al., 2015, Larson et al., 2004, Lukaszewicz et al., 2015, Paulus et al., 2013). It has made it possible to assess different levels of nerve block in regional anaesthesia (Isnardon et al., 2013) and to modulate the use of opioids (Sabourdin et al., 2017) during surgery. Interesting results exist in deeply sedated critically ill and neurocritical ill patients (Lukaszewicz et al., 2015, Paulus et al., 2013, Vinclair et al., 2019).
The recommended use of more superficial levels of sedation and the growing need for more accurate pain assessments determined the performance of this study. We assessed pain in analgosedated critically ill patients and determined the validity and reliability of PDR relative to the Behavioural Pain Scale (BPS).
Methods
Design
This was a diagnostic test study which assessed the pain response of PDR compared to the BPS scale as a reference method.
Participants
We included critically ill patients over 18 years of age, mechanically ventilated, with an initial BPS score of three and a Richmond Agitation and Sedation Scale (RASS) score between −1 and −4. Exclusion criteria were: patients with limitations in the behavioural expression of pain (treatment with muscle relaxants, neuromuscular diseases with motor impairment or severe polyneuropathy ophthalmic pathologies affecting the pupils or the third cranial nerve, Glasgow scores < 6, intracranial hypertension and patients with atropine, clonidine, dexmedetomidine, tramadol, ketamine, adrenaline, calcium antagonists or antiemetic.
Patients meeting these criteria were consecutively selected during the first week of admission to the intensive care unit.
Study protocol
A single protocol per patient was performed. Different intensities of well-tolerated nociceptive stimuli were applied in increasing order of power: non-painful (NP) stimulus, calibrated electrical impulses of 10 mA, 20 mA, 30 mA, and 40 mA, and endotracheal suction (ETA). Gauze was slid over a healthy area of the patient’s forearm as an NP stimulus. ETA was a commonly painful ICU procedure (Puntillo et al., 2014, Robleda et al., 2016).
The BPS scale was a standard tool included in the pain management protocol of the unit and we reported BPS scores before and after each stimulus. The PDR scores were measured automatically by the pupillometer and were taken after the stimulus. We maintained a 5-minute rest between stimulations to allow pupil size values to return to baseline (Fig. 1). Before the protocol, patients remained at rest. They did not undergo potentially painful interventions for at least 1 h. We silenced alarms to avoid possible interference with pupillary responses. We explored a single eye for each patient according to accessibility for measurement and the absence of monocular pathology. The analgesic and sedation regime was kept constant throughout the protocol.
Fig. 1. Study design.
Two researchers conducted the protocol. PDR and BPS measurements were collected simultaneously and remained masked to both investigators.
Outcomes measurements
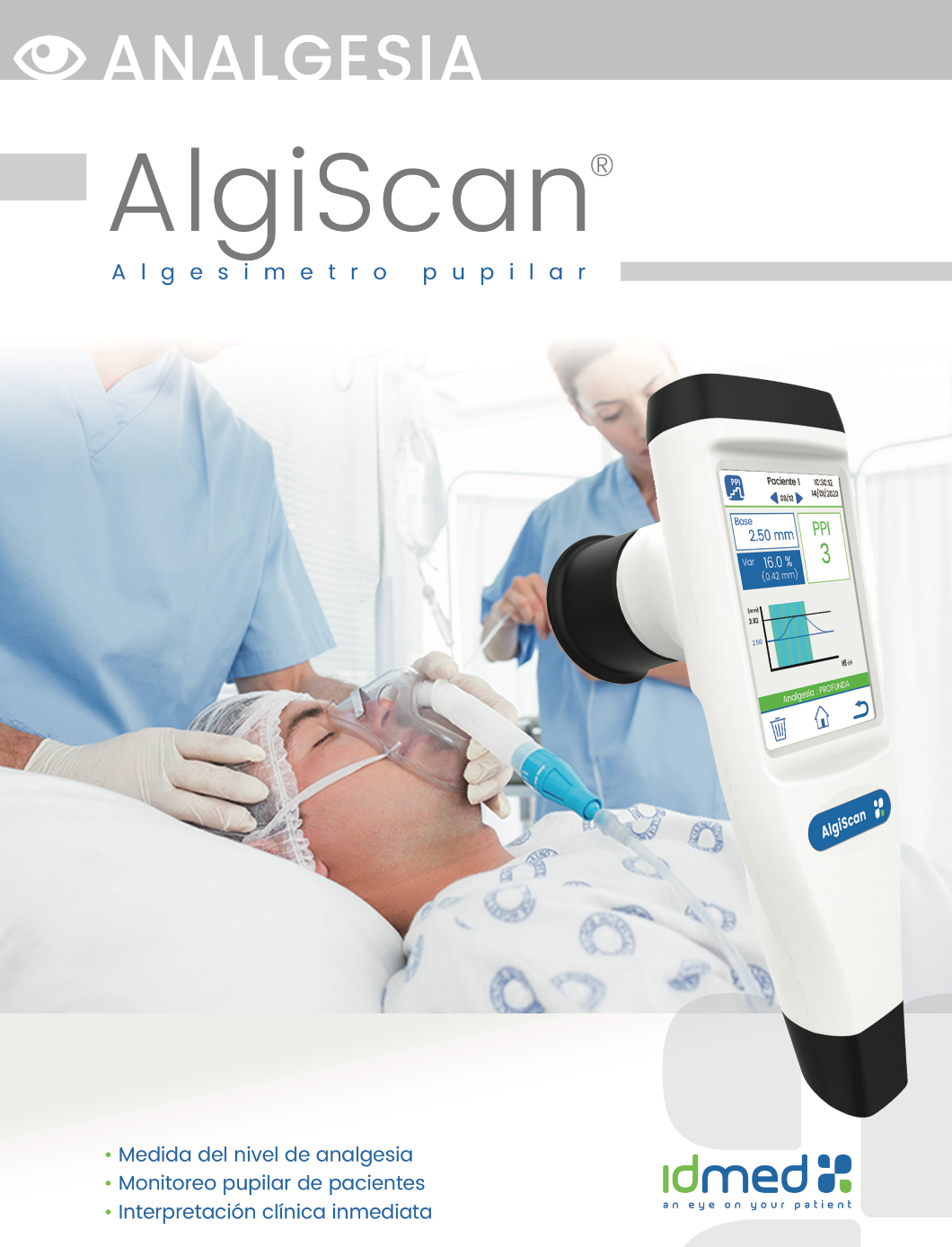
Pupillary measurements were performed with an AlgiScan® portable video pupilometer (ID Company, Marseilles, France). The pupillometer has a video camera, which allows measurements to be performed in the dark and in response to light and calibrated electrical stimuli. It measures the basal pupil diameter and the maximum pupil diameter after the stimulation, with an accuracy of 0.1 mm and a resolution of 0.01 mm. The pupillary dilation reflex (PDR) was collected as a percentage of change in pupillary size secondary to stimulation. We assessed the variation in pupil size during NP and ETA stimulation in DPR mode for 20 s. Calibrated stimuli of 10 mA, 20 mA, 30 mA and 40 mA were delivered through an electrode placed on the ulnar nerve, the inside of the wrist, and clean skin free of any lesion. The Tetanus mode was used with an optimal impedance level identified by the pupilometer. Before measurement, 2–3 s have waited until stabilization of the pupil diameter to the dark environment generated by the eye shield. PDR measurements were performed in strict accordance with the manufactureŕs recommendations.
The pain was assessed using the validated BPS (Ahlers et al., 2010, Aïssaoui et al., 2012, Payen et al., 2001). The BPS scale is a behavioural response assessment scale with three indicators and four items in each, with a range of values from three to twelve points. It is frequently used as a gold standard to assess the accuracy of other tools to measure pain (Fröhlich et al., 2020). Scores equal to or>4 points were considered indicative of pain (Lukaszewicz et al., 2015, Paulus et al., 2013).
Statistical analysis
We calculated the sample size following the method proposed by Flahault et al. (2005). Predicted test sensitivity of 0.95 and a 95 % confidence limit not lower than 0.80 identified the need for approximately 50 measurements with pain according to BPS reported during the protocol interventions.
We described participants’ clinical and demographic characteristics using means and variances, medians and interquartile ranges for continuous variables, and numbers and percentages for qualitative variables and a graphic description of the pain response was made with the two diagnostic tools (BPS and PDR).
A study of diagnostic tests was performed using PDR versus BPS as the reference test. The receiver operating curve (ROC) was plotted, and the area under the curve (AUC) was calculated. We identified the cut-off points showing the highest sensitivity and specificity for each of them. We studied the diagnostic performance based on the Youden index, a summary measurement of the receiver operating characteristic (ROC) curve for the accuracy of a diagnostic test. It evaluates the overall effectiveness of the tool in detecting pain. Values close to 1 represent a perfect diagnostic test. Values relative to 0 indicate that the diagnostic test is ineffective in determining the disease status. We also calculated the positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NPC), and their 95 % confidence intervals (CI). Accuracy was defined as the probability of PDR correctly classifying patients with pain.
We calculated the kappa index (k) between tools to assess the reliability of PDR. The k-value score used is that proposed by Landis and Koch, 1997 (0,00 no agreement; >0,00–0,20 insignificant; 0,21–0,40 low; >0,41–0,60; moderate; 0,61–0,80 strong; 0,81–1,00 very strong).
Finally, we studied the effect of biespectral index (BIS), APACHE II, and age of the patients on pain measured with BPS and PDR. A bivariate analysis was performed using Pearson’s Chi-square and the Mann-Whitney U test. After that, a multivariate analysis was performed using a logistic regression model. BPS was taken as the dependent variable, and PDR as the independent variable. We studied the BPS categorised as BPS < 4 and BPS ≥ 4, and the PDR was analysed as a continuous variable and binary categorical variable according to the previously selected PDR cut-off point. Those variables with a significant relationship with the dependent variable (p < 0.05) and those clinically relevant were included in the model.
A statistical significance level of p = 0.05 was considered in the analysis. Statistical analysis was performed with IBM SPSS Statistics, version 23 for Windows.
Ethics approval
The Clinical Research Ethics Committee of Basque Country approved the project (CEIC-E; internal code: PI2017100). Under Spanish legislation (Royal Decree 223/2004), the study was covered by Professional Civil Liability insurance in any adverse events. Patients’ legal representatives were informed and asked for written consent as a requirement for including patients in the study.
Results
Fifty patients were assessed for eligibility, 18 were excluded, and thirty-one were finally recruited (Fig. 2). One hundred eighty-three measurements were performed; 134 yielded BPS scores < 4, and 49 yielded BPS scores ≥ 4.
Fig. 2. Flowchart of recruited patients.
They were critically ill patients mechanically ventilated with medical or surgical pathologies. The Charlson Comorbidity Index of the patients under study was 3.59 ± 0.55 (Charlson et al., 1994). Twenty-four hours after admission to the ICU, they had an APACHE II value of 21 (16.5–23.5). All patients had continuous analgosedation during the interventions. The general characteristics of the sample and the distribution of medicines and analgesics used are in Table 1.
Table 1. General characteristics of the patients (n = 31).
Age (yr) mean (SD) | 62.9 ± 17.1 |
Sex ratio male/female, n (%) | 19/12 (61.3/38.7) |
Body mass index (kg/m2), median (25-75th percentile) | 27 (24–30) |
APACHE II, median (25-75th percentile) | 21 (16.5–23.5) |
Charlson comorbility Index, mean (SD) | 3.59 ± 0.55 |
ICU mortality, n (%) | 4 (14.8) |
In hospital mortality, n (%) | 2 (7.4) |
Glasgow pre-ETA, median (25-75th percentile) | 15 (15–15) |
BIS, mean (SD) | 63 ± 19.46 |
RASS | −3.39 ± 0.76 |
Admission | |
Acute respiratory failure n (%) | 8 (25.8) |
Postoperative n (%) | 10 (32.2) |
Sepsis n (%) | 9 (29.1) |
Multiple trauma n (%) | 4 (12.9) |
Sedative, analgesic and vasoactive drugs during the procedure | |
Propofol, n (%) | 9 (29.03) |
Dose, (mg/kg/h) mean (SD) | 1.49 ± 0.88 |
Midazolam, n (%) | 16 (51.6) |
Dose (mg/kg/h), mean (SD) | 0.09 ± 0.05 |
Fentanyl, n (%) | 20 (64.5) |
Dose (µg/kg/h), mean (SD) | 1.14 ± 0.74 |
Remifentanil, n (%) | 8 (25.8) |
Dose (µg/kg/h), mean (SD) | 7.05 ± 4.12 |
Morphine, n (%) | 3 (9.6) |
Dose (mg/kg/h), mean (SD) | 1.00 ± 0.44 |
Norepinephrine, n (%) | 22 (71) |
Dose (µg/kg/min), mean (SD) | 0.2 ± 0.13 |
Dobutamine, n (%) | 3 (9.7) |
Dose (µg/kg/min), mean (SD) | 4.10 ± 0.97 |
Data are presented as number of patients (n) and percentage (%), mean and Standard Deviation (SD), Kilogram, milligram and microgram (mg, kg, µg); hour and minute (h, min). APACHE II: Acute Physiology And Chronic Health Evaluation; RASS: Richmond Agitation Sedation Scale; BIS: Bispectral index
Fig. 3 shows that as the BPS score values increase, the percentage of pupil dilation also increases. However, we found pupil variations>20 % corresponding to BPS measurements < 4 indicating no pain. Despite BPS scores in the low range, two measures showed a PDR far superior to the rest. The maximum pain score obtained with BPS was 8 (Fig. 3).
Fig. 3. The pupillary dilation reflex measures and Behavioral Pain Scale scores after stimuli.
No adverse effects were recorded during the use of either diagnostic tool.
Validity
Fig. 4 shows the ROC curve corresponding to the sensitivity and specificity values for each PDR percentage value versus BPS (Fig. 4). The AUC was 0.88 (95 % CI, 0.83–0.94). PDR of 11.5 %, 7.5 %, 5.5 % and 3.5 %, with the best sensitivity and specificity values, were identified as possible cut-off points for assessing pain (Table 2). Among all of them, the cut-off point of 11.5 had the highest values for Youden’s index (0.7), accuracy (81.4; 95 %CI 75.2–86.4), specificity (78.4; 95 % CI 70.6–84.5), PPV (60.3 %; 95 % CI 48.8–70.7) and positive likelihood ratio (4.15; 95 % CI 2.9–5.8). The range of sensitivity values for all cut-off points was 89.8 % to 95.9 %. False positives increased from 21.6 % to 61.9 % as the PDR cut-off point value decreased. The percentage of false positives ranged from 10 to 4 % respectively.
Fig. 4. Area under the indicative curve of pain according to pupillary dilation reflex.
Table 2. Diagnostic ability of cut-off points of pupil size variation to nociception.
PDR 11.5 % | PDR 7.5 % | PDR 5.5 % | PDR 3.5 % | |
Sensitivity (%) | 89.8 (78.2–95.6) | 89.8 (78.2–95.6) | 93.9 (83.5–97.9) | 95.9 (86.3–98.9) |
Specificity (%) | 78.4 (70.6–84.5) | 62.7 (54.3–70.4) | 51.5 (43.1–59.8) | 38.1 (30.3–46.5) |
PPV (%) | 60.3 (48.8–70.7) | 46.8 (37.0–56.8) | 41.4 (32.7–50.7) | 36.2 (28.4–44.7) |
NPV (%) | 95.5 (89.8–98.0) | 94.4 (87.5–97.6) | 95.8 (88.5–98.6) | 96.2 (87.2–99.0) |
FP (%) | 21.6 (15.5–29.4) | 37.3 (29.6–45.7) | 48.5 (40.2–56.9) | 61.9 (53.5–69.7) |
FN (%) | 10.2 (4.4–21.8) | 10.2 (4.4–21.8) | 6.1 (2.1–16.5) | 4.1 (1.1–13.7) |
Accuracy (%) | 81.4 (75.2–86.4) | 69.9 (62.9–76.1) | 62.8 (55.6–69.5) | 53.6 (46.3–60.6) |
PLR | 4.15 (2.9–5.8) | 2.4 (1.9–3.06) | 1.94 (1.6–2.3) | 1.6 (1.4–1.8) |
NLR | 0.13 (0.06–0.3) | 0.16 (0.07–0.36) | 0.12 (0.04–0.36) | 0.1 (0–0.4) |
Youdeńs Index | 0.7 | 0.5 | 0.5 | 0.3 |
Date are shown as a percentage with a 95 % confidence interval (CI); PPV: positive predictive value; NPV: negative predictive value; FP: false positives; FN: false negatives; PLR: positive likelihood ratio; NLR: negative likelihood ratio.
Reliability
PDR showed an overall agreement of 81.4 % with the BPS scale, and the kappa index obtained was 0.6 (Table 3). In 15.8 % of cases, the behavioural scale did not reflect pain (BPS < 4), but the pain was detected by the PDR (PDR ≥ 11.5 %). Only 2.7 % of the discrepancies occurred when the BPS registered pain, but the PDR did not.
Table 3. Agreement between Behavioral Pain Scale and pupil size variation to nociception.
Stimulus | Agreement | Disagreement | ||||
TOTAL | BPS < 4 | BPS ≥ 4 | TOTAL | BPS < 4 | BPS ≥ 4 | |
NP | 29 (93.5) | 29 (93.5) | 0 | 2 (6.5) | 2 (6.5) | – |
10 mA | 29 (93.5) | 29 (93.5) | 0 | 2 (6.5) | 2 (6.5) | – |
20 mA | 24 (77.5) | 22 (71.0) | 2(6,5) | 7 (22.5) | 7 (22.5) | – |
30 mA | 25 (80.7) | 18 (58.1) | 7(22.6) | 6 (19.3) | 3 (9.7) | 3 (9.7) |
40 mA | 18 (64.3) | 7 (25.0) | 11(39.3) | 10 (35,7) | 8 (28.6) | 2 (7.1) |
ETA | 24 (77.4) | 0 | 24 (77.4) | 7 (22.6) | 7 (22.6) | – |
Global (m) | 149 (81.4) | 105 (57.4) | 44(24.0) | 34 (18.5) | 29 (15.8) | 5 (2.7) |
Data are presented as number of patients (n) and percentage (%).BPS: Behavioral Pain Scale; PDR: Pupillary dilation reflex; NP: Non painful estimulus; mA: Miliamperes; ETA: Endotracheal aspiration; m: measurements.
Considering each stimuli separately, we find that the two tools have identical behaviour with a non-painful stimulation and the stimulus of lower intensity (10 mA) (Table 3). We found an agreement in these cases of 93 % and a disagreement of 6.5 %. All these disagreements arose for values of BPS < 4 and PDR ≥ 11.5 %. With higher intensity stimuli, these discrepancies were 22–28 %, except for the 30 mA stimulus, which was 9.7 %. The agreements ranged from 64 to 80 %.
BPS and PDR according to severity level, sedation, and age
The bivariate analysis showed that age (p = 0.884), severity level (p = 0.149), and sedation (p = 0.41) were not associated with BPS. However, BIS and APACHE II were significantly associated with PDR (p < 0.05) on both continuous and categorical analysis, at a cut-off point of 11.5 (Table 4).
Table 4. A bivariate analyses of sedation, severity level, and age on Behavioral Pain Scale and pupil size variation to nociception.
BPS < 4 | BPS ≥ 4 | p | PDR < 11.5 | PDR ≥ 11.5 | p | PDR | p | |
n(%) | n(%) | n(%) | n(%) | P50 (P25-P75) | ||||
BIS ≤ 40 | 20 (14.9) | 5 (10.2) | 0,41 | 19 (17.3) | 6 (8.2) | 0,081 | 3 (2–10,5) | 0,020 |
BIS > 40 | 114 (85.1) | 44 (89.8) | 91 (82.7) | 67 (91.8) | 9 (3,75–21) | |||
Age ≤ 65 | 70 (52.2) | 25 (51.0) | 0,884 | 53 (48.2) | 42 (57.5) | 0,215 | 9 (3–22) | 0,242 |
Age > 65 | 64 (47.8) | 24 (49.0) | 57 (51.8) | 31 (42.5) | 7 (3–19) | |||
APACHE II ≤ 20 | 57 (45.2) | 26 (57.8) | 0,149 | 42 (42.0) | 41 (57.7) | 0,042 | 11 (4–29) | 0,029 |
APACHE II > 20 | 69 (54.8) | 19 (42.2) | 58 (58.0) | 30 (42.3) | 6,5 (3–17) |
Data are presented as number of patients (n) and percentage (%).BPS: Behavioral Pain Scale; PDR: Pupillary dilation reflex; APACHE II: Acute Physiology And Chronic Health Evaluation; BIS: Bispectral index
The OR between BPS and PDR (pupil dilation in percentage, %) in the unadjusted model was 1.094 (1.062–1.127). And taking PDR as a categorized variable with a cut-off point of 11.5 %, the OR was 31,8% (11,579–87,677) (Table 5).
Table 5. Logistic regression analysis adjusted for sedation and severity level.
Univariant analysis | Multivariate Analysis | |||||
OR | IC95% | p | OR | IC95% | p | |
PDR (%) | 1,094 | 1,06–1,12 | <0,001 | 1,095 | 1,06–1,13 | <0,001 |
PDR ≥ 11,5% | 31,862 | 11,57–87,67 | <0,001 | 49,610 | 13,78–178,48 | <0,001 |
OR: Odds ratio; CI: Confidence interval; PDR (%): pupillary dilation reflex expressed as percentage of pupil dilation and analyzed as a continuous variable. PDR ≥ 11.5: pupillary dilation reflex categorized with cut-off point equal or>11.5 % and analyzed as a categorical variable; APACHE II: Acute Physiology And Chronic Health Evaluation; BIS: Bispectral index
After adjusting the model for APACHE II and BIS, we found that these factors did not have an effect on the relationship between BPS and PDR. The OR in the adjusted model with de PDR (%) was 1.095 (1.06–1.13). And taking PDR as a categorized variable with a cut-off point of 11.5 %, the OR was 49.61(13,78–178,48) (Table 5).
Discussion
Detecting pain in critically ill patients requires new indicators that provide more information about their nociceptive response to stimuli. The gold standard for pain assessment is self-referential scales. However, these are not suitable options for sedated mechanically ventilated patients. International institutions recommend using behavioural scales; however, pain in critically ill patients is sometimes not feasible to be assessed through behavioural response. Pupilometer is an objective measure of nociception with good results in different groups of patients. The PDR has been extensively studied in anaesthetized surgical patients, showing differences between blocked and non-blocked sensory areas and greater response in patients with pain (Larson et al., 2004, Constant et al., 2006, Guglielminotti et al., 2015, Isnardon et al., 2013).
Our results suggest that PDR is a diagnostic test with good performance compared to the BPS in patients with RASS between −1 and −4. It could be helpful for the identification of pain in critically ill patients. The AUC was 0.885, the overall agreement was 81.4 %, and the Kappa index was moderate (0.6). In addition, pupillometry detected nociception in 15 % of patients classified as pain-free according to the baseline behavioural scale. A PDR of 11.5 % showed an accuracy of 81.4 % and a sensitivity and specificity of 90 % and 80 %, respectively.
Aissou et al. (2012) obtained a PDR cut-off point of 23 % with a sensitivity, specificity, PPV and NPV of over 90 %. Sabourdin et al. (2018) identified a cut-off point of 32 % with an AUC of 0.758 and a sensitivity and specificity of 0.65 and 0.77, respectively. Nevertheless, they could also evidence the discriminative ability of the pupillary reflex to identify pain in the surgical patient.
Lukaszewicz et al. (2015), and Paulus et al. (2013), observed an increase in PDR in critically ill patients with pain. Recently, Vinclair et al. (2019) reported a correlation between the pupillary pain index (PPI) and the BPS scale. They all showed pupillary reactivity in deeply sedated critically ill patients (RASS −5) and also found a sensitivity and specificity higher than 80 %. However, none of the reviewed papers provides data on the agreement between the two tools for pain diagnosis.
We found that the BIS and the severity of APACHE II were independent of pain response, a favourable condition to implement in the different critically ill patient groups. No studies were found that allowed further discussion and analysis of these results.
Using the behavioural pain scale as a reference tool to assess pain in patients may have limited the results. We detected patients with greater pain responses with PDR than with BPS. The easily changeable of the pupillary size to stimulus could explain this. However, we took some measures to minimise this potential bias: the protocol was performed in a controlled clinical environment, and intense light or auditory stimuli that could generate pupillary responses unrelated to pain were avoided. Similarly, we established a one-hour window with no other procedures (mobilisations, central venous catheter placement, device removal, and wound healing) before measurements to ensure that measures reflected only the response to stimuli administered during the protocol. It is possible that the subjective nature of the behavioural scales and the low behavioural expression in some critically ill patients may have limited the ability of this scale to detect pain.
On the other hand, the miotic effect of opioids might condition the magnitude of the response. Studies show that high doses of opioids can inhibit the pupillary reaction to nociception (Larson and Behrends, 2015). However, in this work, all patients received the recommended opioid doses in each case according to clinical criteria and remained constant during the measurements. And all of them showed some pupil response to pain.
Pupillometry has shown good diagnostic properties of validity and reliability compared to the BPS. And it has the ability to detect nociception in patients without pain according to the reference behavioural scale. So it may allow the detection of pain in patients with limitations in expressing behavioural changes, and in those patients who feel pain, despite staying calm.
Conclusions
The PDR detected nociceptive responses in sedated patients showing adequate validity and reliability compared to the BPS. It is an easy technique to implement in routine clinical practice and could be an optimal tool to monitoring the pain in patients with a limited or abolished behavioural response.
Funding
The study has received an AID in Research Projects from the Basque Government Department of Health with file number 2018111017.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
To those who formed part of the research team collaborating in the recruitment and data collection phase of the PUPIPAIN project for their availability, dedication and time. To the head of investigation service of the Health Research Institute of the hospital for promoting the project. Thanks to the heads of service and the nurse staff of the Intensive Care Units for the support and facilitating attitude shown throughout the project.
Authors’ contributions
University profesor, PhD., RN López de Audícana-Jiménez de Aberasturi Y, PhD., MD. Vallejo-De la Cueva A, and PhD. Parraza-Diez N carried out the conception and design of the study, material preparation and data analysis. All authors contributed to data collection. PhD., RN López de Audícana-Jiménez de Aberasturi Y wrote the first draft of the manuscript and PhD., MD. Vallejo-De la Cueva A, PhD. Parraza-Diez N and MD. Quintano-Rodero A commented on earlier versions of the manuscript. All authors read and approved the final manuscript.
Institute registration
The Clinical Research Ethics Committee of Basque Country approved this study. CEIC-E; internal code: PI2017100.
Trial registration
Phase 1 of the project PUPIPAIN ClinicalTrials.gov Identifier: NCT04078113.
Information
This study was presented as an oral presentation at the 33rd Annual Congress ESICM LIVES Digital 2020 (6th – 9th of December, Madrid, Spain).
Consent to participate
Informed consent was obtained from all individual participants included in the study or their legally authorized representatives.
References
Ahlers et al., 2010
S.J. Ahlers, A.M. van der Veen, M. van Dijk, D. Tibboel, C.A. Knibbe
The use of the Behavioral Pain Scale to assess pain in conscious sedated patients
Anesth. Analg., 110 (1) (2010), pp. 127-133, 10.1213/ANE.0b013e3181c3119e
Aissou et al., 2012
- Aissou, A. Snauwaert, C. Dupuis, A. Atchabahian, F. Aubrun, M. Beaussier
Objective assessment of the immediate postoperative analgesia using pupillary reflex measurement: a prospective and observational study
Anesthesiology., 116 (5) (2012), pp. 1006-1012, 10.1097/ALN.0b013e318251d1fb
Aïssaoui et al., 2012
- Aïssaoui, A.A. Zeggwagh, A. Zekraoui, K. Abidi, R. Abouqal
Validation of a behavioural pain scale in critically ill, sedated, and mechanically ventilated patients
Anesth. Analg., 101 (5) (2005), pp. 1470-1476, 10.1213/01.ANE.0000182331.68722
Barr et al., 2013
- Barr, G.L. Fraser, K. Puntillo, E.W. Ely, C. Gélinas, J.F. Dasta, J.E. Davidson, J.W. Devlin, J.P. Kress, A.M. Joffe, D.B. Coursin, D.L. Herr, A. Tung, B.R. Robinson, D.K. Fontaine, M.A. Ramsay, R.R. Riker, C.N. Sessler, B. Pun, Y. Skrobik, A. College, of Critical Care Medicine,
Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit
Crit. Care Med., 41 (1) (2013), pp. 263-306, 10.1097/CCM.0b013e3182783b72
Chanques et al., 2017
- Chanques, T. Tarri, A. Ride, A. Prades, A. De Jong, J. Carr, N. Molinari, S. Jaber
Analgesia nociception index for the assessment of pain in critically ill patients: a diagnostic accuracy study
Br J. Anaesth., 119 (4) (2017), pp. 812-820, 10.1093/bja/aex210
Charlson et al., 1994
- Charlson, T.P. Szatrowski, J. Peterson, J. Gold
Validation of a combined comorbidity index
- Clin. Epidemiol., 47 (11) (1994), pp. 1245-1251, 10.1016/0895-4356(94)90129-5
Constant et al., 2006
- Constant, M.C. Nghe, L. Boudet, J. Berniere, S. Schrayer, R. Seeman, I. Murat
Reflex pupillary dilatation in response to skin incision and alfentanil in children anaesthetized with sevoflurane: a more sensitive measure of noxious stimulation than the commonly used variables
Br. J. Anaesth., 96 (5) (2006), pp. 614-619, 10.1093/bja/ael073
Devlin et al., 2018
J.W. Devlin, Y. Skrobik, C. Gélinas, D.M. Needham, A.J.C. Slooter, P.P. Pandharipande, P.L. Watson, G.L. Weinhouse, M.E. Nunnally, B. Rochwerg, M.C. Balas, M. van den Boogaard, K.J. Bosma, N.E. Brummel, G. Chanques, L. Denehy, X. Drouot, G.L. Fraser, J.E. Harris, A.M. Joffe, M.E. Kho, J.P. Kress, J.A. Lanphere, S. McKinley, K.J. Neufeld, M.A. Pisani, J.-F. Payen, B.T. Pun, K.A. Puntillo, R.R. Riker, B.R.H. Robinson, Y. Shehabi, P.M. Szumita, C. Winkelman, J.E. Centofanti, C. Price, S. Nikayin, C.J. Misak, P.D. Flood, K. Kiedrowski, W. Alhazzani
Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU
Crit. Care Med., 46 (9) (2018), pp. e825-e873
Flahault et al., 2005
- Flahault, M. Cadilhac, G. Thomas
Sample size calculation should be performed for design accuracy in diagnostic test studies
- Clin. Epidemiol., 58 (8) (2005), pp. 859-862, 10.1016/j.jclinepi.2004.12.009
Fröhlich et al., 2020
M.R. Fröhlich, G. Meyer, R. Spirig, L.M. Bachmann
Comparison of the Zurich Observation Pain Assessment with the Behavioural Pain Scale and the Critical Care Pain Observation Tool in nonverbal patients in the intensive care unit: A prospective observational study
Intensive Crit. Care Nurs., 60 (2020), Article 102874, 10.1016/j.iccn.2020.102874
Gélinas and Johnston, 2007
- Gélinas, C. Johnston
Pain assessment in the critically ill ventilated adult: validation of the Critical-Care Pain Observation Tool and physiologic indicators
Clin. J. Pain, 23 (6) (2007), pp. 497-505, 10.1097/AJP.0b013e31806a23fb
Guglielminotti et al., 2015
- Guglielminotti, N. Grillot, M. Paule, F. Mentré, F. Servin, P. Montravers, D. Longrois
Prediction of movement to surgical stimulation by the pupillary dilatation reflex amplitude evoked by a standardized noxious test
Anesthesiology, 122 (5) (2015), pp. 985-993, 10.1097/ALN.0000000000000624
Isnardon et al., 2013
- Isnardon, M. Vinclair, C. Genty, A. Hebrard, P. Albaladejo, J.F. Payen
Pupillometry to detect pain response during general anaesthesia following unilateral popliteal sciatic nerve block: a prospective, observational study
Eur. J. Anaesthesiol., 30 (7) (2013), pp. 429-434, 10.1097/EJA.0b013e32835f0030
Landis and Koch, 1977
J.R. Landis, G.G. Koch
The Measurement of Observer Agreement for Categorical Data
Biometrics, 33 (1) (1977), p. 159
Larson and Behrends, 2015
M.D. Larson, M. Behrends
Portable infrared pupillometry: a review
Anesth. Analg., 120 (6) (2015), pp. 1242-1253, 10.1213/ANE.0000000000000314
Larson et al., 2004
M.D. Larson, P.D. Berry, J. May, A. Bjorksten, D.I. Sessler
Latency of pupillary reflex dilation during general anesthesia
- Appl. Physiol., 97 (2) (2004), pp. 725-730, 10.1152/japplphysiol.00098.2004
Lukaszewicz et al., 2015
A.C. Lukaszewicz, D. Dereu, E. Gayat, D. Payen
The relevance of pupillometry for evaluation of analgesia before noxious procedures in the intensive care unit
Anesth. Analg., 120 (6) (2015), pp. 1297-1300, 10.1213/ANE.0000000000000609
Martini et al., 2015
C.H. Martini, M. Boon, S.J. Broens, E.F. Hekkelman, L.A. Oudhoff, A.W. Buddeke, A. Dahan
Ability of the nociception level, a multiparameter composite of autonomic signals, to detect noxious stimuli during propofol-remifentanil anesthesia
Anesthesiology, 123 (3) (2015), pp. 524-534, 10.1097/ALN.0000000000000757
Paulus et al., 2013
- Paulus, A. Roquilly, H. Beloeil, J. Théraud, K. Asehnoune, C. Lejus
Pupillary reflex measurement predicts insufficient analgesia before endotracheal suctioning in critically ill patients
Crit. Care, 17 (4) (2013), p. R161, 10.1186/cc12840
Pavone et al., 2021
K.J. Pavone, J. Jablonski, P.Z. Cacchione, R.C. Polomano, P. Compton
Evaluating Pain, Opioids, and Delirium in Critically Ill Older Adults
Clin Nurs Res., 30 (4) (2021), pp. 455-463, 10.1177/1054773820973123
Payen et al., 2001
J.F. Payen, O. Bru, J.L. Bosson, A. Lagrasta, E. Novel, I. Deschaux, P. Lavagne, C. Jacquot
Assessing pain in critically ill sedated patients by using a behavioral pain scale
Crit. Care Med., 29 (12) (2001), pp. 2258-2263, 10.1097/00003246-200112000-00004
Puntillo and Naidu, 2016
K.A. Puntillo, R. Naidu
Chronic pain disorders after critical illness and ICU-acquired opioid dependence: two clinical conundra
Curr Opin Crit Care., 22 (5) (2016), pp. 506-512, 10.1097/MCC.0000000000000343
Puntillo et al., 2010
K.A. Puntillo, S. Arai, N.H. Cohen, M.A. Gropper, J. Neuhaus, S.M. Paul, C. Miaskowski
Symptoms experienced by intensive care unit patients at high risk of dying
Crit. Care Med., 38 (11) (2010), pp. 2155-2160, 10.1097/CCM.0b013e3181f267ee
Puntillo et al., 2014
K.A. Puntillo, A. Max, J.-F. Timsit, L. Vignoud, G. Chanques, G. Robleda, F. Roche-Campo, J. Mancebo, J.V. Divatia, M. Soares, D.C. Ionescu, I.M. Grintescu, I.L. Vasiliu, S.M. Maggiore, K. Rusinova, R. Owczuk, I. Egerod, E.D.E. Papathanassoglou, M. Kyranou, G.M. Joynt, G. Burghi, R.C. Freebairn, K.M. Ho, A. Kaarlola, R.T. Gerritsen, J. Kesecioglu, M.M.S. Sulaj, M. Norrenberg, D.D. Benoit, M.S.G. Seha, A. Hennein, F.J. Periera, J.S. Benbenishty, F. Abroug, A. Aquilina, J.R.C. Monte, Y. An, E. Azoulay
Determinants of procedural pain intensity in the intensive care unit. The Europain® study
Am. J. Respir. Crit. Care Med., 189 (1) (2014), pp. 39-47
Rababa and Al-Rawashdeh, 2021
- Rababa, S. Al-Rawashdeh
Critical care nurses’ critical thinking and decision making related to pain management
Intensive Crit. Care Nurs., 63 (2021), Article 103000, 10.1016/j.iccn.2020.103000
Rawal et al., 2017
- Rawal, S. Yadav, R. Kumar
Post-intensive Care Syndrome: an Overview
J Transl Int Med., 5 (2) (2017), pp. 90-92, 10.1515/jtim-2016-0016
Robleda et al., 2016
- Robleda, F. Roche-Campo, L. Membrilla-Martínez, A. Fernández-Lucio, M. Villamor-Vázquez, A. Merten, I. Gich, J. Mancebo, E. Català-Puigbó, J.E. Baños
Evaluación del dolor durante la movilización y la aspiración endotraqueal en pacientes críticos [Evaluation of pain during mobilization and endotracheal aspiration in critical patients]
Med Intensiva., 40 (2) (2016), pp. 96-104, 10.1016/j.medin.2015.03.004
Rotondi et al., 2002
A.J. Rotondi, L. Chelluri, C. Sirio, A. Mendelsohn, R. Schulz, S. Belle, K. Im, M. Donahoe, M.R. Pinsky
Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit
Crit. Care Med., 30 (4) (2002), pp. 746-752, 10.1097/00003246-200204000-00004
Sabourdin et al., 2017
- Sabourdin, J. Barrois, N. Louvet, A. Rigouzzo, M.L. Guye, C. Dadure, I. Constant
Pupillometry-guided Intraoperative Remifentanil Administration versus Standard Practice Influences Opioid Use: A Randomized Study
Anesthesiology, 127 (2) (2017), pp. 284-292, 10.1097/ALN.0000000000001705
Sabourdin et al., 2018
- Sabourdin, T. Giral, R. Wolk, N. Louvet, I. Constant
Pupillary reflex dilation in response to incremental nociceptive stimuli in patients receiving intravenous ketamine
- Clin. Monit. Comput., 32 (5) (2018), pp. 921-928, 10.1007/s10877-017-0072-5
Schittek et al., 2021
G.A. Schittek, H. Simonis, H. Bornemann-Cimenti
Pain, nausea, vomiting, thirst, cold, the challenge of well-being in post-operative patients
Intensive Crit. Care Nurs., 66 (2021), Article 103090, 10.1016/j.iccn.2021.103090
Vinclair et al., 2019
- Vinclair, C. Schilte, F. Roudaud, J. Lavolaine, G. Francony, P. Bouzat, J.L. Bosson, J.F. Payen
Using Pupillary Pain Index to Assess Nociception in Sedated Critically Ill Patients
Anesth. Analg., 129 (6) (2019), pp. 1540-1546, 10.1213/ANE.0000000000004173
1
ORCID: 0000-0001-5703-2706.
2
ORCID: 0000-0003-4094-6506.
3
ORCID: 0000-0002-6391-2897.
4
ORCID: 0000-0002-5902-0351.
© 2022 The Authors. Published by Elsevier Ltd.